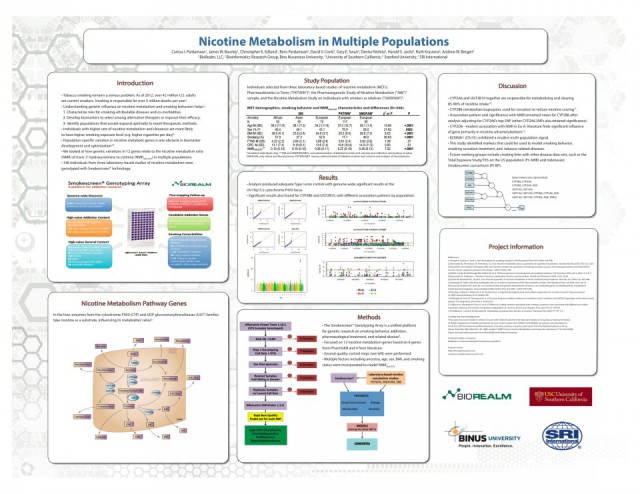
Nicotine Metabolism In Multiple Populations

Authors:
Carissa I. Pardamean*, James W. Baurley, Christopher K. Edlund, BioRealm; Bens Pardamean, Bina Nusantara University; David V. Conti, University of Southern California; Gary E. Swan, Stanford University; Denise Nishita, Harold S. Javitz, Ruth Krasnow, Andrew W. Bergen, SRI International
Abstract:
The overall smoking rate has been reduced significantly through tobacco control, public health, and pharmacological interventions. Metabolic enzyme variation, as well as other patient and environmental characteristics, influences smoking behavior, treatment success, and risk of related diseases. Population specific variation in nicotine and other tobacco smoke constituent metabolic genes contributes to challenges in developing and optimizing interventions. We applied novel molecular genetic technology, public pharmacological data, and three laboratory-based studies of nicotine metabolism with oral and/or venous administration of labeled nicotine and cotinine to provide an integrated approach to modeling nicotine metabolism in multiple populations.
260 individuals (160, 48, and 52 self-identified European, African, and Asian American ancestry individuals) were genotyped using the Smokescreen platform, a targeted genotyping array for addiction research. The model predicted natural log transformed nicotine metabolite ratio (NMR) of trans-3’-hydroxycotinine to cotinine, and included ancestry represented by principal components, age, sex, BMI, and smoking status. Analysis demonstrates adequate Type I error control with genome-wide significant results at the chr19q13.2 cytochrome P450 locus (minimum meta-analysis p-values provided; P=1.11E-11) and variants within nicotine metabolism pathway genes such as AOX1 (P= 0.011), FMO1 (P=0.020), POR (P=0.002), UGT2B10 and UGT2B17 (p-values < 0.036). Additionally, we observed an emerging multi-ethnic meta-analysis peak on chr10q (P=7.02E-7). This study indicates a promising start to finding population-specific and nonspecific biomarkers for tobacco-related diseases, smoking cessation treatment, and smoking behavior in future studies. Our results also have the potential to create links with other tobacco-related disease datasets and discovery and treatment oriented research groups (e.g., Indonesia’s Smokescreen consortium).
Funding:
This project has been funded in whole or in part with: Federal funds from the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN271201300004C, by research and development funds from SRI International and Bina Nusantara University, and by a research project grant from the National Institute on Drug Abuse (Rockville, MD), DA033813 (PI: AWB), entitled: “DMET Genes, Nicotine Metabolism and Prospective Abstinence.
Justification:
This research will identify genetic factors predictive of nicotine metabolism in multiple populations, leading to development of models that may enable better predictions on smoking behavior, treatment success, and attributable disease.
Corresponding Author:
James Baurley, PhD, Data Scientist, BioRealm LLC, 19330 Rim of the World Dr, Monument, CO 80132, United States, Phone: 855-777-3256, Email: baurley@biorealmresearch.com
This article is presented in 2015 Society for Research on Nicotine and Tobacco 21th Annual Meeting, February 25-28, 2015, Philadelphia, Pennsylvania, USA.